how many valence electrons are in lithium|How to Find the Valence Electrons for Lithium (Li) : iloilo Lithium has a single electron in the second principal energy level and so we say that . Hotel O Pino, O Pino: See 184 traveller reviews, 66 candid photos, and great deals for Hotel O Pino, ranked #2 of 11 B&Bs / inns in O Pino and rated 4 of 5 at Tripadvisor. Flights Holiday Rentals Restaurants Things to do .
PH0 · Valency of Lithium
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence Electrons Chart for All Elements
PH3 · Lithium Valence Electrons
PH4 · How to Find the Valence Electrons for Lithium (Li)?
PH5 · How to Find the Valence Electrons for Lithium (Li)
PH6 · How many valence electrons does lithium have?
PH7 · 3.10: Valence Electrons
2 Horas de música clásica de Mozart especialmente elegida para bebés. La música ha sido elegida cuidadosamente para ayudar a los bebés a relajarse y dormir m.MOA Brgy Kibaghot Sample - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. This memorandum of agreement is between the Kibaghot Elementary School and the Barangay of Kibaghot to collaborate on projects that will improve the school and create a safe learning environment. The Barangay agrees .
how many valence electrons are in lithium*******Valence electrons for representative elements. Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Li: 1s 2 2s 1 (the electron in the 2s energy level is the valence electron) Beryllium has two .
Given that 7 Li is 7.0160 amu and 6 Li is 6.0151 amu, and their percent .Lithium has a single electron in the second principal energy level and so we say that . Mar 23, 2023
Find the valence of lithium and other elements in this table based on the bond . There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium has in . Thus, lithium-ion (Li+) has eight valence electrons. Li+ valency is not zero like noble gas as their outermost shell has eight electrons. when a lithium atom loses one electron, Li+ ion is produced .
Lithium has a 1 valence electron. Here, we have mentioned the Lewis structure of the lithium.How many valence electrons does lithium have? Answer: Lithium has 1 valence electron. Explanation: Valence electrons are the electrons present in the outermost .
There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium ha.Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, . Valences of the Elements Chemistry Table. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Answer: Lithium has 1 valence electron. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Lithium (Li), with an atomic number of 3, has an electron configuration of 1s 2 2s 1.In this configuration, the 2s subshell contains 1 electron, which is the valence electron.
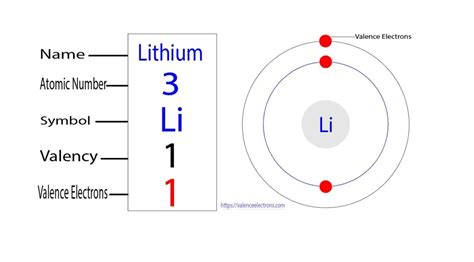
The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. . Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and . The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: \[\mathbf{Li}\mathbf{\cdot}\nonumber \] Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of .How many electrons does a lithium atom have? Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. . The last shell of lithium has an unpaired electron, so the valency of lithium is 1, and the last shell has a total of an electron, so the valence electrons of lithium .
For instance, lithium (Li ) has three electrons: two fill the 1 s orbital, and the third is placed in the 2 s orbital, giving an electron configuration of 1 s 2 2 s 1 . Neon ( Ne ), on the other hand, has a total of ten electrons: two are in its innermost 1 s orbital and eight fill the second shell—two each in the .The next element is lithium, with Z = 3 and three electrons in the neutral atom. . How many valence electrons are found in the ground state electron configuration for Element 114? Answer: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 2 ; 4 valence electrons (from 7s and 7p orbitals. Also .
How to Find the Valence Electrons for Lithium (Li) The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order .how many valence electrons are in lithium How to Find the Valence Electrons for Lithium (Li) The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order .Li + has zero valence electrons. Explanation: The electronic configuration of Li is 1s22s1 . It means it has 2 elec. View the full answer. Step 2. Unlock. Unlock. Step 3.
how many valence electrons are in lithiumLi + has zero valence electrons. Explanation: The electronic configuration of Li is 1s22s1 . It means it has 2 elec. View the full answer. Step 2. Unlock. Unlock. Step 3. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the . Figure 2.4.1 2.4. 1: Shell diagram of lithium (Li) atom. The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s2 2 s2. Now that the 2 s subshell is filled, electrons in larger atoms start filling the 2 p subshell. Thus, the electron configurations for the next six atoms are as follows: Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second principal . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
Read 2,015 galleries with tag cbt on nhentai, a hentai doujinshi and manga reader.Download CAD block in DWG. General car parking plan. (721.43 KB) General car parking plan. Search. Log In; Deutsch; English; Español; Français; Portugues; . Curved covered warehouse for bus garage. dwg. 2.9k. Parking lot cross section of a parking lot. dwg. 2.8k. Parking for 400 cars on 4 levels. dwg. 8.6k. Combi station with gas station. rvt.
how many valence electrons are in lithium|How to Find the Valence Electrons for Lithium (Li)